
Cois Coiribe: Where does your passion for medical research stem from?
Peter Doran: At age 13, my mother, Ann Doran was diagnosed with breast cancer and passed six months later in 1989. Given all the research carried out since, had my mother been diagnosed today, her prognosis would not have been so bleak. This demonstrates how trials work and how they have changed outcomes for patients. Sadly, my father Billy was diagnosed with a brain tumour just two years after my mother’s death, and passed away after three months.
Experiencing the death of both my parents was emotionally challenging and triggered a desire to understand the factors that contributed to their passing – to drive forward research as a meaningful pursuit. I often think that if my mother was diagnosed today, her outcome would be different, however my father’s outcome sadly wouldn’t be much better. In setting up the first Institute for Clinical Trials at the University of Galway, my mission is to improve health for all by supporting the development of better and safer treatments, as well as diagnostics, for disease management and prevention. University of Galway is rising to this challenge, bringing the best scientific minds together to tackle the problems that our patients and their families face on a daily basis.
We are accumulating increasing evidence that patients who are cared for in a research–active environment have better outcomes. For example, a comprehensive analysis of NHS trusts showed that those trusts that received more research funding and had more research activity, had lower mortality. (1)
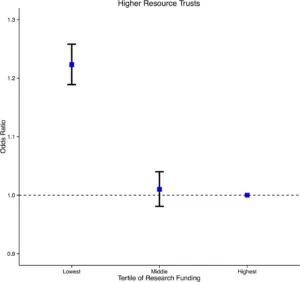
Higher Resource Trusts
Risk adjusted odds ratio of inpatient death in English NHS Trusts according to scaled CCRN funding. Trusts in the lowest funding range had significantly higher mortality relative to the highest funded trusts. © 2015 Ozdemir et al.
CC: Why is it so important for this region to set up a clinical trials institute?
PD: Clinical trials are integral to effective healthcare. They are the means by which we evaluate new treatments to improve outcomes for patients. Think of common drugs that patients use on a daily basis to treat illnesses such as high blood pressure or diabetes; these have all travelled the path of clinical research in order to be evaluated to be safe and competent. Add to this specialised healthcare, new vaccines, greater awareness of rare diseases and improved accessibility to healthcare.
The last decades have undoubtedly been the golden age of biomedical discovery. From the sequencing of the human genome to the development of gene editing, scientists have made discoveries, developed technologies and expanded understanding in ways that were unimaginable 50 years ago. These discoveries place us in an unprecedented time to impact patients. By translating basic findings to personalised, refined therapies, we stand at the cusp of an era of progress which will likely see outcomes for patients transformed. I have no doubt that we are now entering a golden age of medicine, where the untreatable becomes manageable, where side effects are eliminated and where quality of life is transformed. Clinical trials are the vital next step in translating findings to impact for patients.
The launching of this institute embodies the University’s ambition to be at the cutting edge of clinical research, advancing science, finding cures and improving the health of the nation. We hope to transform the clinical research landscape in the West of Ireland, with a comprehensive end-to-end programme that supports the delivery of clinical trials, from initial concept to reporting.
The region itself will greatly benefit from the institute as a focal point for sustained, mutually beneficial partnerships with academic and industry partners. The aim is to position Galway as the life sciences centre of Ireland and contribute to economic growth and regional development. Put simply, we will prioritise science that creates impact.
CC: This institute will be the first of its kind in the country. What is your vision for the research programme, and more broadly, patient care?
PD: Our vision is to translate research into impact in a way that is inclusive and driven by need. We are developing a place where discoveries are made and diseases are cured. In every decision we make, we consider our core pillars: developing (building partnerships), delivering (excelling in trial delivery), innovating (striving towards the trials of the future), influencing (shaping policy) and training (advancing our workforce).
Our institute is positioned to rise to the challenges that people face today. We are articulating an ambitious plan to enhance our contribution to society. We recognise the complexities of these problems and are committed to aligning our assets and promoting interdisciplinary research to achieve progress. We will avoid silos, and remain focused.
CC: Which issues in healthcare will you be focusing on?
PD: The research ambition of the Institute is driven by patient need. We will prioritise conditions that have the biggest impact on national and regional populations. Consider the field of Oncology. Oncology clinical trial starts reached historically high levels in 2020, up 60% from 2015, and focused mostly on rare cancer indications. This growth has continued. More than 700 companies are involved in cancer trials. Across the globe, an estimated 19,500 cancer clinical trials are underway, propelled in good part by an increase in global spending on anti-cancer drugs, which is projected to reach US $269 billion by 2025.
Cancer trials will further expand and refine over the coming years and we here at the University of Galway will be part of this transformation. Cancer care in the future will likely evolve to bio-specific immunotherapy – directed by antibody-drug conjugates, titrated to specific patients from the results of liquid biopsy – driving a dramatic change in outcomes. I truly believe we are at a time of unprecedented opportunity.