
University of Galway alum, Dr Martin Walsh OBE has spent 30+ years studying the structure of biological molecules. In his work at Diamond Light Source, Oxfordshire, Dr Walsh uses X-ray crystallography to image a range of biological macromolecules from proteins and nucleic acids to viruses at the molecular level. The technique relies on generating crystals of the macromolecules which are then illuminated with X-rays to determine their atomic structures.
At the outset of the COVID-19 pandemic, the skills of Dr Walsh and his team were diverted towards early drug discovery in the race to address the worldwide health crisis. Now in 2023, he has been named an Officer of the Most Excellent Order of the British Empire (OBE) for his services to Science during COVID-19.
Cois Coiribe: We would like to extend our congratulations on your recognition as OBE for services in science. Can you tell us more about your research at Diamond Light Source?
Dr Martin Walsh: It’s an honour to be awarded this OBE; it was a complete shock, to be honest. Diamond Light Source is a facility that provides access to state-of-the-art instrumentation across all sciences, from physics to chemistry and biology. Over 14,000 registered scientists come here to use Diamond, the UK’s national synchrotron facility. The OBE really recognises all of us here at Diamond, and what we have achieved for science during COVID and more generally.
You’re probably wondering what a synchrotron is; you can think of it as a very high-powered microscope. We use the whole spectrum of light, from X-rays all the way through to UV and infrared, but primarily X-rays because they are very penetrating and versatile. They are scattered, absorbed and diffracted by matter. So, we can use a range of techniques to understand the structure and makeup of for example many biological materials, probe the dynamics of these systems and understand their function. Synchrotron radiation is extremely versatile so researchers can also exploit it to study new materials to aid the development of e.g., better batteries and more efficient data storage solutions. Through the range of techniques available at Diamond, we try to understand the fundamentals of materials and thereby, improve systems – eventually providing information for more applied research that benefits the general public.
A synchrotron is essentially a particle accelerator. We accelerate electrons to close the speed of light at high energy (3 Giga electron volts, 3 GeV). The electrons are bent in a circular orbit by a series of magnets losing energy in the form of synchrotron light which is then funnelled to dedicated experimental stations called beamlines. Synchrotron radiation was first observed in 1947 in Wisconsin, U.S.A and X-rays were discovered at the end of the 19th century by Wilhelm Roentgen. Synchrotrons were initially developed for use in high energy physics, looking at the fundamentals of atomic particles. Then during the late 50s and early 60s, people came to appreciate the characteristics of the synchrotron radiation for other techniques. Some scientists started using these facilities developed for high energy physics experiments parasitically and showed the potential of using synchrotron radiation more widely in a dedicated manner.
This led the development of what were termed second generation synchrotron sources to use this kind of radiation across a broad range of science (1). In my field, structural biology, synchrotrons have been essential in driving forward key research for the past 30 years.
Synchrotrons are a powerful tool for us to observe the structural makeup of the cell, and to look at the structure of individual proteins and viruses. Structural biology came into its own at the beginning of the 1980s with the development of molecular biology techniques. These technologies started to impact in the mid ’80s. Molecular biology and recombinant protein production (cloning of genes responsible for encoding the information to produce a protein) in particular. I finished my degree at University of Galway in 1989, just as these had started to take off. With more reliable synchrotrons coming online in the mid-’90s, structural biology quickly became a powerful way of driving development in new drugs for various diseases.

CC: How has your research involved global collaboration?
MW: In the case of the pandemic, collaboration was unplanned and evolved naturally. I had worked with Chinese collaborators on proteins from the original SARS virus in 2002, which is very similar to SARS-CoV-2. Due to the small number of deaths, there was unfortunately no incentive from big pharma or governments to provide adequate funding for drug discovery targeting the 2002 SARS virus. The irony is – had they provided that funding, we would likely have had antivirals ready to use on SARS-CoV-2. So, for a small amount of early investment, they could have potentially saved many lives and avoided the massive economic impact of the SARS-CoV-2 pandemic. So, you can see why there is now an emphasis on preparedness for future potential pandemics.
In the past, we worked with colleagues in China in particular Prof Zihe Rao (Beijing & Shanghai) and Prof Haito Yang (Shanghai). There is a synchrotron in Shanghai which is similar in capabilities to Diamond and their groups did an amazing job in starting structural work immediately as the SARS-CoV-2 virus, first isolated in Wuhan, was sequenced.
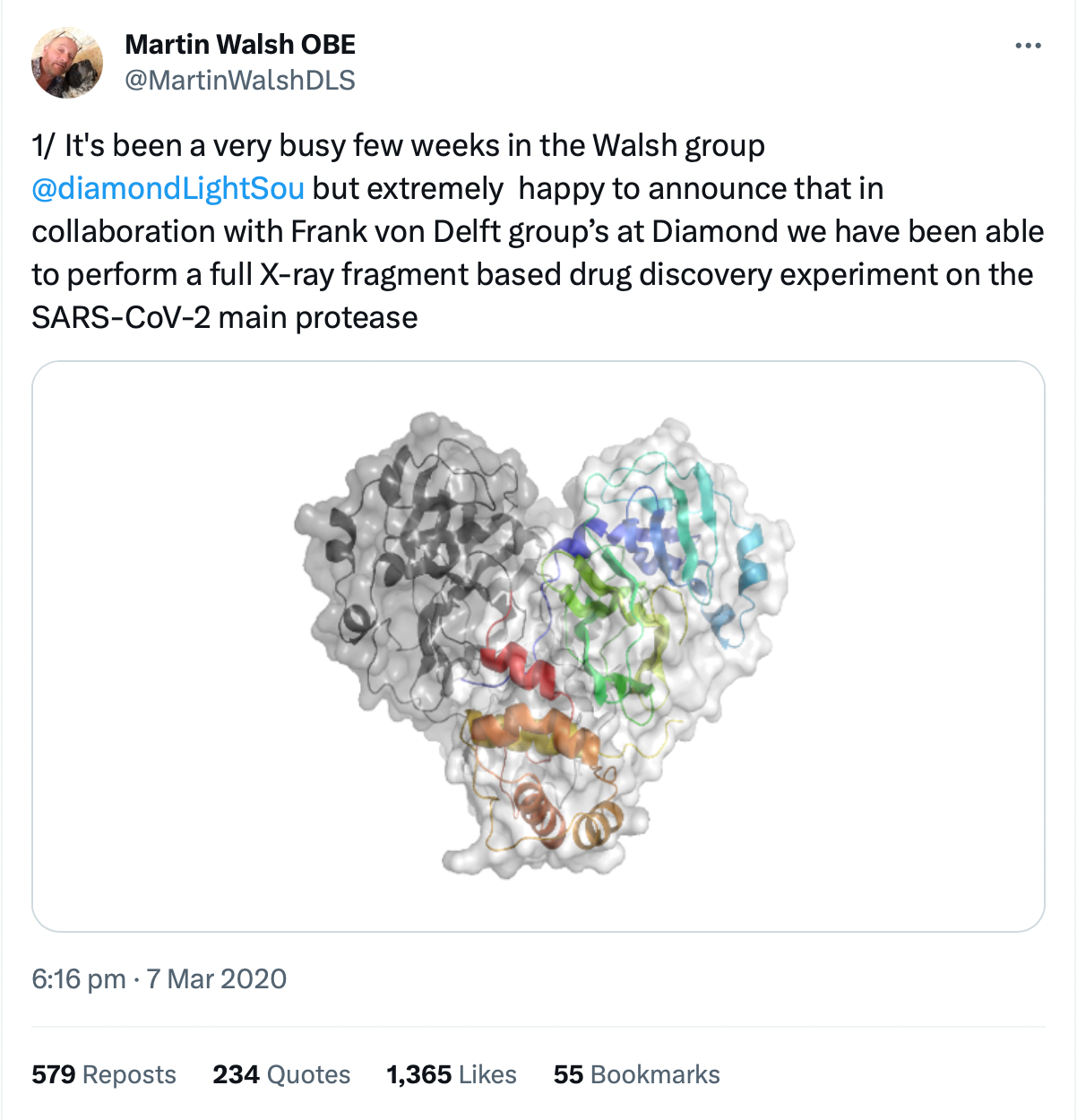
Each protein has a specific sequence of amino acids. Once the genome of the virus was sequenced, the Chinese group were able to overproduce proteins encoded by the viral genome for structural studies. They were able to quickly target specific proteins that would provide crucial structural data for drug and vaccine development. They selected a key viral protein that is essential for viral replication and made crystals of this protein, which is an enzyme known as the main protease; proteases chop up a protein at specific sites in their amino acid sequence.
They were then able to determine the structure of the protein using X-ray crystallography. Amazingly, they managed to solve the structure in early January 2020, but their synchrotron was scheduled to shut down for a maintenance period and so, they contacted us to see if we could help. We said, “Yes,” just as China went into complete lockdown at the end of January. They couldn’t FedEx their samples to us with this lockdown in place. So, we ended up having to redo that work.
We were lucky. If we had started a few days later, we wouldn’t have achieved our goal in time because a lot of the laboratory supplies, especially for molecular biology, are sourced from China. With the lockdown in place, you couldn’t get consumables essential to the experiments. Avoiding that obstacle, we managed to clone and produce the protein by the 14th of February. On Valentine’s Day 2020, we had our first protein crystals – reproducing the work of the Chinese group with their advice on what had worked for them. From then on, it was a roller coaster. There were many challenges to overcome but luck was on our side and we overcame what could have been major problems.
After the experiment, we decided to release all the information immediately. Developing a new antiviral can take 10–20 years; we didn’t expect to move things forward at that speed but we had diverted all our resources to this project from this very early stage before we even went into lockdown in the UK. While everyone was sat at home in April of 2020, I sent out a Tweet telling everyone what we had done. It may not have been viral by influencer standards but it certainly was viral for me as a scientist. All I could hear was the, “Ding, ding, ding” of my phone. We received a massive response.
Of the companies keen to engage, the first was PostEra, a start-up that uses AI machine learning to accelerate the design of new compounds to be taken forward for drug discovery. Working with PostEra and another group from Israel, we made the data more accessible through an interactive database. Additional Tweets were published asking medicinal chemists to look at the data and suggest compound designs. We also got other academics and industry partners involved.
One partner, a Kiev-based chemical company called Enamine had a major library of compounds and the expertise to synthesis new chemical designs proposed by the chemists who engaged in the call. With Enamine, we started to synthesise designs from 350 chemists across the world, analysing their interaction with our protein. It was a special situation that saw us expand from a team of four at Diamond to a network of academics and industry leaders across the globe. Our unplanned project had developed into a series of compounds which were credible drug-like compounds that were serious candidates for taking to clinical trials, all of which happened in approximately 18 months.
We now have $67 million in funding from the NIH (the U.S.A National Institutes of Health) for an official three-year project coined ASAP, involving many original partners. That funding will allow us to apply a similar methodology to the range of viruses that could potentially start another pandemic. One major thing to come from COVID is awareness around pandemic preparedness. Horizon Europe, for example, are now funding researchers to work together to make educated guesses on viruses that could potentially cause the next pandemic.
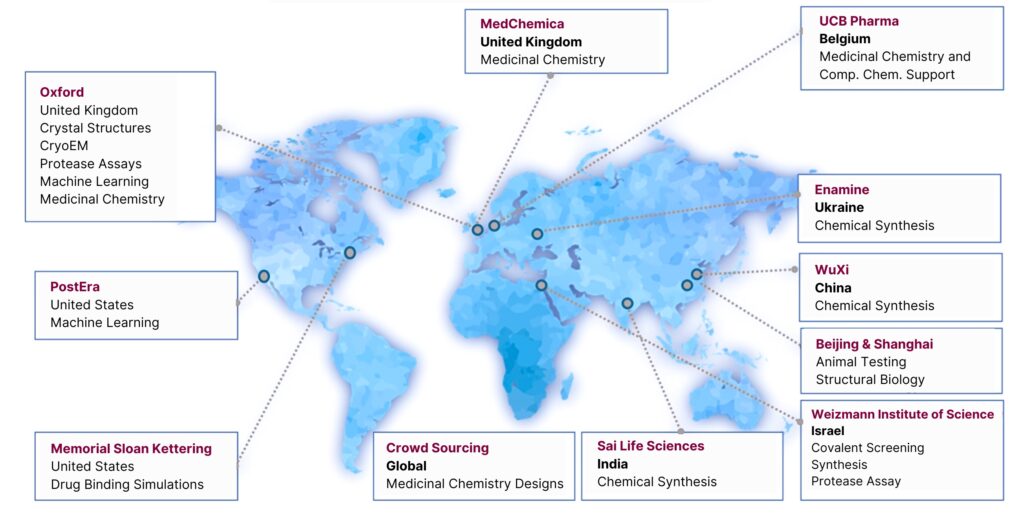
Moonshot and beyond – a global effort. Image courtesy of Dr Martin Walsh OBE.
CC: You have taken an open-access approach in your work on the proteases of SARS-CoV-2. How important was this decision and did you face any challenges?
MW: Doing open science when you want to deliver a commercial drug is challenging, but the COVID Moonshot project shows that is possible and will continue through our NIH-funded ASAP centre, which uses a combination of experimental techniques and AI to develop antivirals against a plethora of potential pandemic-causing viruses. A lot of research on third-world diseases go unfunded because there is no market incentive in developing countries. From our perspective as scientists, the project was easy to start but when it comes to the nitty gritty of licensing and clinical trials, there is some work involved. We‘re now taking this pilot project forward with DNDI (Drugs for Neglected Diseases Initiative), a non-profit NGO that helps to fast track new therapeutics for neglected diseases and their expertise was key for taking forward the COVID Moonshot initiative with funding from the Wellcome trust, a charitable organisation based in the UK which provides support to scientists in the life sciences.
CC: You’re often observing life at a cellular level. Has this shaped your outlook on the world?
MW: I first got into crystallography at University of Galway, after some convincing from Prof Patrick McArdle who has a way with words and of sharing his enthusiasm for chemistry. At the time, everyone told me I was completely mad because it was the very beginning of protein crystallography in Ireland, and there was no money. But understanding the synchrotron and having the opportunity to do experiments there – really was a pull.
I started looking at things that people had never seen before, understanding them at the atomic level. I don’t think this necessarily changed my general outlook on life, but it is cool to look at something so small and be able to understand how it works. Now the field is changing again. We can do amazing things through cryo-electron microscopy (Cryo-EM), where electrons are used instead of X-rays. With cryo-EM we can now look at the structure of proteins in their natural cell environment on the nanoscale (10-9 M or 1 billionth of a metre!).
Exploiting molecular biology techniques, we are able to clone proteins and purify them in high enough quantities to then crystallize them and use X-ray crystallography to determine their three-dimensional structures where we can distinguish individual atoms. With electron microscopy, we can bypass the need to grow crystals and can even take single cells, freeze them, and use a technique whereby you look at the structure of the protein(s) in their natural cellular environment at resolutions close to atomic resolution.
Other groups here in Diamond worked with the Oxford Vaccine Group which developed the AstraZeneca vaccine. The AstraZeneca vaccine uses the spike protein of the SARS-CoV-2 virus as the antigen to generate an immune response. At Diamond, to ensure the vaccine generated the active structural conformation, researchers used cryo-EM to visualise the structure of the spike protein produced by the vaccine in the cell. The spike protein is presented on the outside of the virus structure and is essential for the virus to penetrate the cell. The spike protein has a dynamic structure with different conformations before and after it fuses with cells. Through electron microscopy, we were able to show at high resolution that the spike protein produced by the Oxford/Astra Zeneca vaccine had the right conformation when produced in the cell to raise a strong immune response to SARS-CoV-2. Being able to see viruses in the cell at the nanoscale is a new frontier for structural biology.
CC: It must have been surreal to look at the virus up close while the pandemic unfolded around you. How can this help us prepare for future pandemics?
MW: Our director here at Diamond, Sir Dave Stuart, was looking at new variants as they emerged, characterising the binding of antibodies to these variants in near real time – it was completely crazy. They were identifying the variants of concern very quickly, and assessing the changes in the spike protein structure that allowed the virus to evade the antibody response. Investment in Diamond since its establishment in 2002 has been around £1.3 billion, but we’ve generated far more impact than that. It was recently estimated that we have had a £2.6 billion impact on UK science and economy since 2007. We just received funding of just over half a billion from the UK Government and the Wellcome trust to upgrade the whole facility by 2030. That will prove essential in preparing for the next pandemic and for other challenges like antimicrobial resistance.
Even though the pandemic was massive, people can quickly forget. Sometimes it takes someone falling off a bridge for us all to decide to fix the railing. We can’t simply react; we have to prepare for these things. And we need to work hard to raise public awareness on the impact of continued investment in science. If we had never received the support and training here to work on the first SARS pandemic in 2002, we would never have achieved the same progress with the work we did on SARS-CoV-2. To bring together good scientists and solve global challenges, you need consistent funding.
CC: As a kid growing up in Westport, did you show an early interest in science?
MW: People often have wonderful answers to this question – “At the age of five, something happened and I thought, ‘Oh yes, I want to be a scientist!’” I never related to this, for me it was more a journey of fortunate encounters or accidents. I think everyone finds science fascinating. I do remember getting a chemistry set from Santa at eight though, which was great fun.
I started secondary school in in England before moving back to Westport. In the UK, I was given my first taste of doing practical science experiments as well as woodwork and metalwork classes. I had only spent my first term of secondary school in England before moving back and ending up in sixth class (due to the different school system). By the time I started Irish secondary school, I was excited to get back to doing science practicals, but it wasn’t until I entered fifth year, I finally got to do hands-on experiments in science as well as building projects through doing building construction for the leaving cert.
Back in the 80s, everyone I knew wanted to be an electronic engineer. I didn’t know what I wanted to do but I liked science. Prof Frank Gannon ran a Masters in biotechnology course at University of Galway. In fifth year at secondary school, I wrote to the department, and they actually replied. I was surprised to hear back from them, and that always stuck with me. They recommended a basic degree in the sciences before moving onto doing something like biotechnology through a Master’s degree.
So, in the end I decided to do the B.Sc. degree at Galway which was a great decision. In first year science, all the sciences were taught, and I took a real interest in chemistry but for the more focused areas of biology i.e. microbiology and biochemistry, there were a short series of lectures and for microbiology these were given by Prof Peter Smith, and he was exceedingly charismatic. So, I ended up focusing on chemistry microbiology and maths in second year science ultimately taking Chemistry as my major and microbiology as my minor subject and hence my windy road from not doing chemistry at school to ending up as a chemist.
By the time I finished up at university, I was having so much fun in Galway and enjoying the science. A bunch of us thought, “Who wants a job at 21?” So, being keen to do research we did a Master’s or PhD instead. You shouldn’t say those kinds of things, but many of us from the class of ’89 have remained in academia. So, it wasn’t such a bad thing! I didn’t have a vision – just a rocky road influenced by the people I came into contact with. Fortunately, Prof Patrick McArdle, Prof Des Cunningham and Prof Tim Higgins had started a crystallography group in the chemistry department and had visited a world-leading protein crystallography lab at York university in the UK during the summer of 1989 to learn what was needed to do protein crystallography. Their enthusiasm convinced me to do my PhD in protein crystallography.
I still keep in contact with the University of Galway and people in Mayo. Strangely, at that time, I was convinced that Ballina had the highest concentration of trained crystallographers in Ireland. If there was an all-Ireland competition for crystallographers, Mayo might still win.
SDGs discussed in this article:
Profiles

Dr Martin Walsh OBE is Deputy Director of Life Sciences at Diamond Light Source. Walsh is also a Research Group Leader at the Research Complex at Harwell (RCaH). He joined Diamond in January 2009 from the Medical Research Council (MRC), France. After graduating from University of Galway (formerly UCG) with a first class Honours degree in Chemistry in 1989, he remained at University of Galway for his PhD which used X-ray crystallography to characterise the bacterial protein flavodoxin.
In 2009, Walsh joined Diamond Light Source with responsibility for life science research. He was made an Officer of the Most Excellent Order of the British Empire (OBE) for services to Science during Covid-19, in the King's first Birthday Honours List in June 2023.